- Research
- Open access
- Published:
Humic acid potentiometric response patterns: out-of-equilibrium properties and species distribution modeling
Chemical and Biological Technologies in Agriculture volume 2, Article number: 17 (2015)
Abstract
Background
Negentropy and entropy fluctuations, which are concepts of out-of-equilibrium thermodynamics, were considered in the development of a simple potentiometric titration method for the study of natural complex systems, in this case humic acids.
Results
The method allows, besides the obtainment of traditional titration curves, the observation of response patterns for the out-of-equilibrium evolution throughout the titration pH range, at each point of the titration. Also, two humic acid species distribution models are proposed and interpreted.
Conclusions
The obtained potentiometric out-of-equilibrium response patterns (graphical method) are correlated with the negentropic organizational/structural properties of the sample and provide information on its relation with the natural environment.

Potentiometric out-of-equilibrium response patterns suggest that major supramolecular humic acid (HA) transformations occurs at neutral pH, revealing the complexity of these highly organized structures which directly affect ecosystem thermodynamics.
Background
In recent decades, interest in natural systems, such as oceans, soils, and the biosphere, has been increasing considerably, and in the associated scientific research areas (e.g., biogeochemistry and soil science) significant challenges are encountered due to the complexity of the factors related to these systems [1, 2]. In this context lie the complexity of humic substances, their structural/organizational properties, and their functional relations with the negentropic properties of ecological networks and the health of entire ecosystems. To address these issues, several methodologies have been developed and, as expected, new phenomena and new questions have arisen [3].
At present, it is partially accepted that natural humic substances are composed of a mixture of small molecules and macromolecules (biomolecules, proteins, carbohydrates, etc.), and diverse proposals for the organization of humic materials have been proposed [4].
Among the existing methodologies for the study of humic substances, spectroscopic and potentiometric methods are important contributors to the advances in this field [5, 6]. Specifically, potentiometric titration has been used intensively as a cheap, accessible, easy to handle, and highly informative intermediate technique, permitting the researcher to characterize natural complex systems, such as humic substances, from the thermodynamic perspective of equilibrium constants and to quantify/qualify the proton exchange properties of the samples [7–11].
Also, in the investigation of natural complex systems (integer or raw complex systems) using potentiometric methods, out-of-equilibrium thermodynamic states can be observed in terms of seconds, minutes, or hours [12]. It is interesting to note that this is not a property of all chemical systems (for example, small molecules or homogeneous solutions of pure substances), but out-of-equilibrium stable thermodynamic states are characteristic of complex, highly organized heterogeneous (negentropic) systems, such as humic substances, soils, microbiological systems, networks, root systems, and many others found in natural environments [8–16]. A simple and important relation (for our approach and other related strategies) has been identified in modern out-of-equilibrium thermodynamics [15], which involves free energy and entropy:
since d totalS = d i S + d e S, where d i S and d e S are the entropy variation of the internal system and the external system (surroundings), respectively. When a system is perturbed (d e S < 0), or when a system occupies an out-of-equilibrium state close to equilibrium (or pseudo-equilibrium), it tends to dissipate free energy with increasing entropy as follows:
and the thermodynamic evolution drives the system toward the nearest pseudo-equilibrium state, usually through exponential free energy decay (e.g., Fig. 1).
These simple theoretical considerations on out-of-equilibrium thermodynamics permit the use of potentiometry for the investigation of perturbed systems during potentiometric titrations based on the well-known relation between potential and free energy:
(ΔG H+ is the free energy related to a H+ inhomogeneities in hydrogen ionic potential measurements) and the obtainment of out-of-equilibrium response patterns for natural complex systems, which can be correlated with the organizational/structural properties of the studied systems and their relations and functions in the natural environment (e.g., the role of humic substance in soils and ecosystems). In this study, we used these concepts to construct out-of-equilibrium response patterns through the potentiometric titration of humic acid samples and then correlate these graphical patterns with the complete titration curve and structural-chemical composition of this important component of natural systems, as described in the following sections.
Methods
Representation of pH stabilization curves—“the graphical method”
Since the free energy, related to a H+ inhomogeneities, of the system (after titrant addition) tends to dissipate through spontaneous reactions (in our case, proton exchange between the humic acid system and the aqueous environment), the slow pH stabilization curves (which are kinetically measurable) after each titrant addition adopt a commonly observed exponential evolution over time, tending toward a pseudo-equilibrium state or quasi-stable pH conditions, as observed in Fig. 1.
For the fitting of the slow pH stabilization curves, we used the following asymptotic exponential equation:
where y(x) is the measured pH, x is time t, a is the quasi-stable pH value when x tends to infinity, b is the variation of y(x) (ΔpH) from x = 0 to x = ∞ and c is the exponential coefficient which, multiplied by b, defines the shape of the pH versus time variation curve and is related to the out-of-equilibrium negentropic stability of the studied sample [12].
Species distribution calculations
The analysis of the complete titration curve and the model calculations were performed using the Best 7 program (calculated models were obtained with resulting error function σ fit < 0.02) [17] in order to determine the number of moles of the components and their respective protonation constants. Considering the complex structure of humic acids, in this study, two slightly different ways to approach and model this system were investigated. The first approach (named M1) was based on the model described in Costa et al. [18], in which carboxylic, phthalic, phenolic, catechol, and salicylic groups are considered to be the main protonable components of humic acids (Scheme 1). Also, this model assumes significant alterations in the protonation constants of the carboxylic and catechol groups due to intra- and intermolecular interactions (see Table 1). The second approach involved the proposal of a new modeling strategy (named M2) using the same groups mentioned above (adopting protonation constants constrained to standard data [19], with minor variations) but with the adoption of a new species proposed as a supramolecular protonable structure, as expected for humic acids at near-neutral pH (hypothetical structure proposed in Scheme 1). It is important to remark that the “supramolecular” term was used in reference to the subsystems which present slow proton exchange kinetics, as can be seen in Figs. 1 and 2, probably composed by the combinations of carboxylic and hydroxy aromatic groups, involving hydrogen bonding, electrostatic interactions, and Van der Waals interactions. The results were compared and correlated with the out-of-equilibrium response patterns as discussed below.
Results and discussion
Considering that our interest is focused on the out-of-equilibrium properties (which relate to the system stability against perturbations) and species distribution of humic acids, the proposed titration methodology represents an appropriate potentiometric experiment since the two aspects mentioned above could be investigated in the same titration procedure.
The results for the humic acids (Fig. 3) show a smooth titration curve, and a suitable model is needed for the species distribution calculation (as expected due to the complexity of the sample and as previously noted in the literature) [6–11].
In this regard, the first model M1 was employed to calculate protonable species distribution (carboxylic, phthalic, phenolic, catechol, and salicylic groups) and the results were then compared with those previously published by Costa. [18]. As can be seen in Table 1, good agreement between the two sets of results was verified and interesting features appeared under neutral pH conditions. It can be observed that despite being composed of the above-cited oxygenated groups, the humic acid sample presents a high buffering capacity at neutral pH, suggesting that the structure and organization of this substance have distinct features compared with the isolated molecules or functional groups (extremely simplified models should not be considered as good options to humic experimental or theoretical studies, neither pedagogically nor scientifically). This was well represented in the application of our first calculation model M1 through the increase in the pKa of the carboxylic group from 4.56 to 6.38 and the decrease in the pKa1 of the catechol group from 9.23 to 7.92 (compared with standard values [19], Table 1) suggesting that complex structural phenomena, actually composed by an incommensurable mixture of structural/functional groups, influence the proton dissociation properties of humic acid, providing a model where carboxylic groups exist in a distinct molecular environment when compared with single molecules and pure dissolved substances. Similar phenomena can be considered in the case of the catechol groups, where near-positioned protonated groups should cause a decrease in the observed macroscopic deprotonation constant.
In the second model approach (M2) to protonable species distribution calculations, using an additional protonable species, which is proposed as a supramolecular structural combination, and maintaining the pKa values of oxygenated groups without major alterations in relation to standard protonation constants [19], (Table 1), a good fit was obtained for the experimental curve (Fig. 3), using coherent pKa values for the oxygenated groups and an average apparent pKa of 6.65 for the proposed supramolecular species, as can be seen in the species distribution diagram (Fig. 4) and Table 1. This is in good agreement with the maximum at pH 6.7 in Fig. 2 (top), which will be discussed below (in relation to the humic acids out-of-equilibrium time response pattern with each titrant addition). The difference between models M1 and M2 in relation to the calculated number of moles for the proposed species suggests that carboxylic groups are the major participants in the supramolecular interactions and that the other oxygenated groups participate to a lesser extent, representing approximately 26 % of the total buffering capacity of the humic acid sample studied (Table 1). Figure 4 shows the species distribution diagram for model M2 in terms of the percentage of each component.
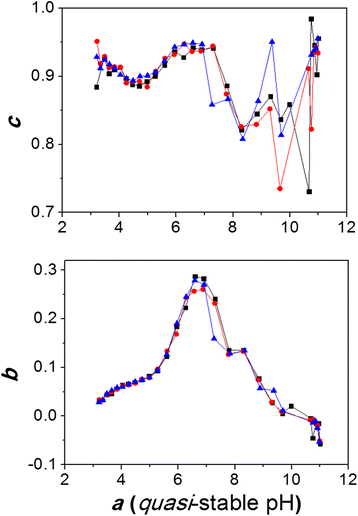
Out-of-equilibrium potentiometric response patterns for parameters b (bottom) and c (top) as functions of a (see Eq. 5). Color lines represent the triplicate results
As previously mentioned, the methodology used in this study involved the investigation of the out-of-equilibrium pH/time response patterns for all points of the potentiometric titration (Figs. 1 and 2), simultaneously with the traditional titration curve (Fig. 3). Since relatively large amounts of titrant (0.1 mL of 0.1029 mol.L−1 HCl) were used in the present titration studies, slow pH stabilization processes seem to be amplified and out-of-equilibrium states became easily measurable. The basic information obtained through this method relates to the stability of the studied system against perturbations (titrant addition) in terms of the parameters b and c in Eq. 5, which relate to the pH variation (ΔpH) and the relative pH variation rate, respectively. Setting parameters b and c as functions of parameter a (stabilization pH or quasi-stable pH at each point of the titration), the stability response patterns in Fig. 2 were constructed. The maximum at pH 6.70 observed in Fig. 2 (top) may be related to a highly stable humic acids subsystem which is capable of responding to perturbations by increasing the pH by approximately 0.3 pH units above the initial pH (when an aliquot of HCl is added). Furthermore, this subsystem could be directly related to the supramolecular species proposed in the second species distribution approach M2 or, alternatively, to the phenomena responsible for the significant alteration in the calculated carboxylic and phenolic pKas in the first species distribution approach M1 (Table 1). Ester reactions can also be involved in the observed slow kinetic processes [20, 21]. These information are in well agreement with previous potentiometric and calorimetric studies that suggest that high energy-consuming chemisorption processes (around 38 kJ.mol−1) may dominate the buffering properties of humic acids at neutral pH [22].
A maximum, or plateau, in the region of pH 6.4–6.9 can also be observed in Fig. 2 (bottom), which indicates that the relative variation in the pH after perturbation occurs more slowly in this pH region than in other pH regions (around 5 or 8), since the major pH variation is found at pH 6.7, as seen for parameter b in Fig. 2 (top). Also, it is important to note that the high variance of parameter c at basic conditions (Fig. 2, bottom) is related to small or negligible pH variations observed after perturbation in these basic pH regions as can be seen (Fig. 2, top). Together, these results provide important information regarding the proton exchange properties of the complex system of humic acids. Also, through the observation of the slow pH stabilization curves at each point of the titration, we can propose that humic acids can adopt different structures (possibly with a transition between coiled forms under acid conditions and relaxed forms under basic conditions) with critical changes occurring at around pH 6.7, where a high consumption of protons is observed over long time periods (at least 20 min, Figs. 1 and 2). Finally, since the apparently soluble humic acid is actually a suspension of nanometric (around 400 nm) particles, it should be expected that slow proton exchange may occur at a larger extent in continuous perturbed natural conditions.
Experimental
After adequate homogenization of the commercial humic acid sample (Sigma Aldrich) the potentiometric experiment was performed as follows.
The potentiometric system consists of a thermostatized electrochemical cell at 25 °C, a continuous N2 flux, a glass electrode connected to a pH meter (precision 0.001 pH units), and a Gilmont burette (precision 0.01 mL). The electrode was calibrated through the titration of 40 mL of 0.01 mol.L−1 HCl (0.1 mol.L−1 KCl) with 0.0971 mol.L−1 CO2-free KOH. All the solutions were prepared, and all experiments were performed using boiled ultrapure water.
In the electrochemical cell, 51 mg of well-homogenized humic acid sample was dissolved in 40 mL of water (ionic strength 0.1 mol.L−1 KCl). After 1 h of continuous stirring (dissolution pH 9.1), the pH was raised to 11 by adding 1.1 mL of 0.097 mol.L−1 CO2-free KOH. Immediately after the KOH addition, the variation in the pH was recorded at 0, 0.5, 1, 2, 4, 8, and 16 min in order to observe the response of the system in relation to perturbation. After basification, titration with 0.1 mL aliquots of 0.1029 mol.L−1 HCl was initiated and the pH was measured over 16 min at the above-mentioned time intervals for all titrant additions, resulting in a characteristic pH versus time stabilization curve for each point of the titration. It is important to note that the rapid initial acidification of the aqueous medium was not kinetically monitored (usually occurring in less than 10 s) and that the out-of-equilibrium observations are related to kinetically measurable slow proton exchange processes (from 0.5 to 16 min) that contribute to the total buffering capacity of the humic acid sample in study. The complete titration involved 30 aliquot additions until the pH value reached 3.2 (Fig. 3). The experiments were carried out in triplicate.
Conclusions
In this research, we investigated the use of potentiometric techniques for the study of complex samples and we found that the stabilization of the pH over time for each point of the titration can provide interesting information on the organization of humic acids. Applying this simple investigative methodology, we demonstrated that this substance has a high buffering capacity and stability against perturbations, such as the addition of strong acids and strong bases. This is probably due to the capacity for structural reorientation and a highly stable organization or negentropy, mainly at neutral pH. These characteristics are strongly related to soil stability/fertility and the health of ecosystems under different conditions, such as in the highly energetic, biodiverse natural/forest soils, and also soils of alternative sustainable agricultural systems. We propose that our simple methodology is appropriate for use in studies on a wide variety of complex systems, including other humic substances, soils, root systems and, importantly, integer samples or raw complex systems [12]. Lastly, it is important to note that in the study of complex systems the use of potentiometry (considering out-of-equilibrium thermodynamics) is proposed to contribute with transdisciplinar (e.g., agroecology) investigation and the development of socially-scientifically-technologically relevant research focused on intermediate and accessible technologies [1, 12, 23–25].
References
Naveh Z (2001) Ten major premises for a holistic conception of multifunctional landscapes. Landscape and Urban Planning 57:269. http://dx.doi.org/10.1016/S0169-2046(01)00209-2
Capra F (2006) A teia da vida: uma nova compreensão científica dos sistemas vivos. Cultrix, São Paulo
Sutton R, Sposito G (2005) Molecular structure in soil humic substances: the new view. Environ. Sci. & Tech. 39:9009. http://dx.doi.org/10.1021/es050778q
Piccolo A (2001) The supramolecular structure of humic substances. Soil Science 166:810. http://dx.doi.org/10.1097/00010694-200111000-00007
Yang Y, Chase HA (1998) Applications of Raman and surface-enhanced Raman scattering techniques to humic substances. Spectroscopy Letters 31:821. http://dx.doi.org/10.1080/00387019808007402
Drosos M, Jerzykiewicz M, Deligiannakis Y (2009) H-binding groups in lignite vs. soil humic acids: NICA-Donnan and spectroscopic parameters. J. Colloid Interf. Sci. 332:78. http://dx.doi.org/10.1016/j.jcis.2008.12.023
Ritchie JD, Perdue EM (2003) Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 67:85. http://dx.doi.org/10.1016/S0016-7037(02)01044-X
Martell AE, Motekaitis RJ (1992) Determination and Use of Stability Constants. Viking, Texas
Milne CJ, Kinniburgh DG, Tipping E (2001) Generic NICA-Donnan model parameters for proton binding by humic substances. Env. Sci. Technol. 35:2049. http://dx.doi.org/10.1021/es000123j
Drosos M, Leenheer JA, Avgeropoulos A, Deligiannakis Y (2014) H-binding of size- and polarity-fractionated soil and lignite humic acids after removal of metal and ash components. Env. Sci. Pollut. Research 21:3963. http://dx.doi.org/10.1007/s11356-013-2302-9
Baidoo E, et al. (2014) Potentiometric studies of the acid–base properties of tropical humic acids. Geoderma 18:217–218. http://dx.doi.org/10.1016/j.geoderma.2013.10.020
de Almeida VR, Szpoganicz B (2013) Proton exchange in mycelium/water system: transdisciplinary out-of-equilibrium thermodynamic approach using potentiometric titration. Open J. Phys. Chem. 3:189. http://dx.doi.org/10.4236/ojpc.2013.34023
Kazakov S, Bonvouloir E, Gazaryan I (2008) Physicochemical characterization of natural ionic microreservoirs: Bacillus subtilis dormant spores. J. Phys. Chem. B 112:2233. http://dx.doi.org/10.1021/jp077188u
Michaelian K (2011) Entropy production and the origin of life. J. Modern Phys. 2(6A):595. http://dx.doi.org/10.4236/jmp.2011.226069
Kondepudi D, Prigogine I (1998) Modern Thermodynamics: From Heat Engines to Dissipative Structures. Wiley, Chichester, UK
Seders LA, Fein JB (2011) Proton binding of bacterial exudates determined through potentiometric titrations. Chem. Geol. 285:115. http://dx.doi.org/10.1016/j.chemgeo.2011.03.017
Motekaitis RJ, Martell AE (1982) BEST—a new program for rigorous calculation of equilibrium parameters of complex multicomponent systems. Can. J. Chem. 60:2403. http://dx.doi.org/10.1139/v82-347
Costa TG, et al. (2008) Equilibrium studies of the interaction of Zn(II) and Cu(II) ions with humic acid by potentiometric titration, FT-IR, and fluoroscence spectroscopy. South Braz. J. Chem. 16:1. http://www.sbjchem.he.com.br/jornal/2008.pdf
Martell AE (1977) Critical Stability Constants. Plenum, New York
Almendros G, Sanz J (1989) Compounds released from humic acids upon BF3-MeOH transesterification. Sci. Total Environ. 51:81–82. http://dx.doi.org/10.1016/0048-9697(89)90110-1
Nebbioso A, Piccolo A (2011) Basis of a humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules 12:1187. http://dx.doi.org/10.1021/bm101488e
Pertusatti J, Prado AGS (2007) Buffer capacity of humic acid: thermodynamic approach. J. Colloid Interface Sci. 314:484. http://dx.doi.org/10.1016/j.jcis.2007.06.006
Bateson G (1987) Steps to an Ecology of Mind: Collected Essays in Anthropology, Psychiatry, Evolution and Epistemology. Jason Aranson, London
Maturana H, et al. (1997) A ontologia da realidade. Humanitas, Belo Horizonte
Rifkin J, Howard T (1980) Entropy: A New World View. Viking, New York
Acknowledgements
We thank CNPq (Brazilian National Counsel of Technological and Scientific Development), the Federal University of Santa Catarina (UFSC), and the Chemistry Department (DQ-UFSC) for financial support and infrastructure.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The publication of this article is supported by BioMed Central open access publisher.
Authors’ contributions
VRA conducted potentiometric measurements, whole data treatment, and development of representation models. BS contributed on potentiometric measurements and species distribution modeling. Both authors read and approved the final manuscript.
Authors’ information
VRA is a graduate of Bachelor in Chemistry and Bachelor in Technological Chemistry, licensed in Chemistry Education (2010), holds a master in (Bio)Inorganic Chemistry (2012), and Ph.D. candidate on Inorganic Chemistry at the Universidade Federal de Santa Catarina, Brazil. He is also a current participant in the “Science without Borders” Brazilian program at Université Libre de Bruxelles, Belgium and is involved in the thesis project “Transdisciplinarity and Ecology in Chemistry.” BS is a Ph.D. in Chemistry by the Texas A&M University (1984) and full professor of the Chemistry Department of Universidade Federal de Santa Catarina, Brazil. He has large experience on chemical equilibrium in aqueous solution and metal complexation with organic ligands, melanins, and humic substances.
An erratum to this article is available at http://dx.doi.org/10.1186/s40538-015-0046-0.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Almeida, V.R., Szpoganicz, B. Humic acid potentiometric response patterns: out-of-equilibrium properties and species distribution modeling. Chem. Biol. Technol. Agric. 2, 17 (2015). https://doi.org/10.1186/s40538-015-0042-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-015-0042-4